International Pediatric Thrombosis Network
working together to improve care of pediatric patients with thrombosis
The International Pediatric Thrombosis Network is a group of pediatric thrombosis experts whose ultimate goal is to improve care of all children with thrombosis worldwide through international research collaboration and education.
The IPTN is initiated by the Pediatric and Neonatal Hemostasis and Thrombosis Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis.
It enables international research collaboration in pediatric thrombosis by facilitating prospective international observational studies using the Throm-PED registry. In addition, the IPTN serves as a platform for the Throm-PED Clinical Trial Network, a network of pediatric thrombosis centers with clinical trial experience and infrastructure to effectively conduct both investigator-initiated academic trials and industry-sponsored trials.

IPTN in numbers
(per June 19, 2025)
Throm-PED Registry
Members of the IPTN register consecutive pediatric patients with venous and arterial TEs in the Throm-PED registry. The basis registry consists of age at diagnosis of TE, gender, type, location, risk factors, and treatment.
IPTN Projects
Each member of the IPTN may propose sub-studies using the Throm-PED registry. Each approved IPTN project has its own scientific board, which is responsible for the design and execution of the IPTN project.
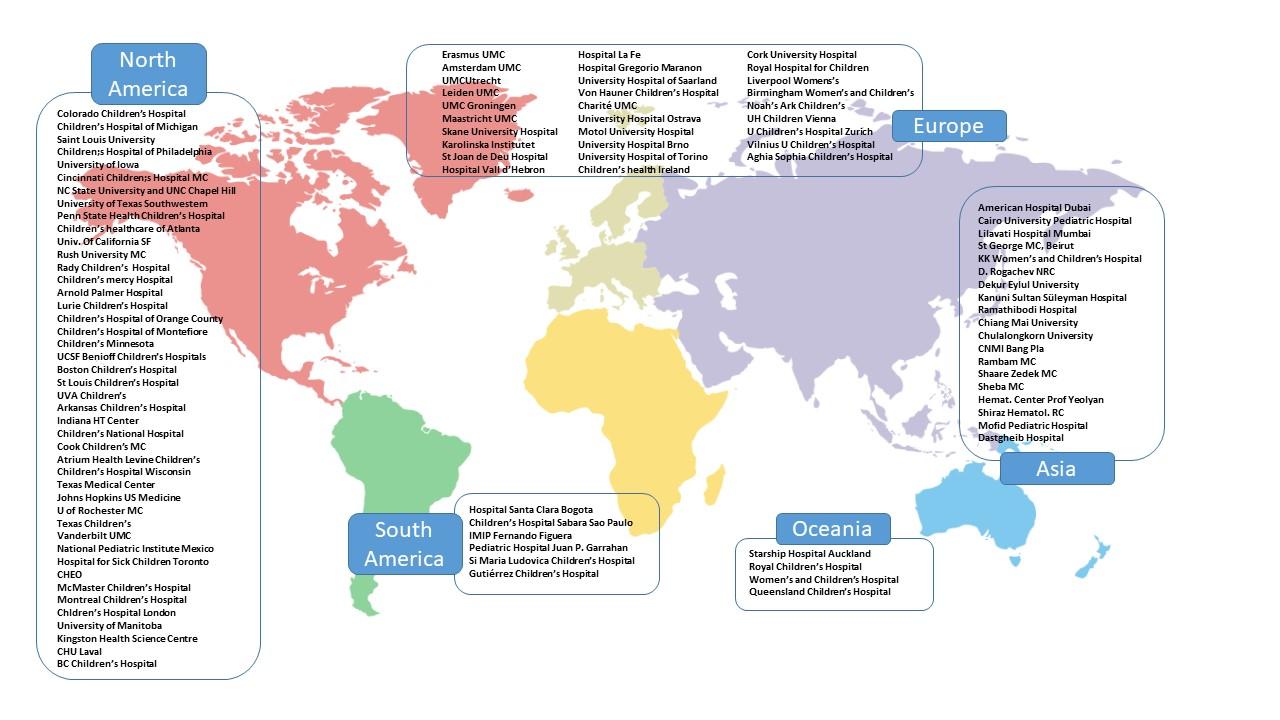
IPTN worldwide: participating centres